Polyvinyl Alcohol (PVA) is a synthetic polymer that has gained significant attention across various industries due to its unique properties and versatility. PVA’s applications are broad, from textiles to pharmaceuticals, and its role in modern manufacturing and technology is indispensable. To fully appreciate the impact of PVA, it’s essential to understand the science behind this remarkable material—specifically its structure and properties, which make it so valuable in a wide range of applications.
What is Polyvinyl Alcohol?
Polyvinyl Alcohol (PVA) is a water-soluble polymer synthesized from polyvinyl acetate (PVAc) through a process known as hydrolysis. The polymerization of vinyl acetate produces PVAc, which is then treated with an alcohol (usually methanol or ethanol) to replace the acetate groups with hydroxyl groups, resulting in PVA. The degree of hydrolysis, which refers to the extent to which the acetate groups are replaced, plays a crucial role in determining the final properties of PVA.
The Structure of Polyvinyl Alcohol
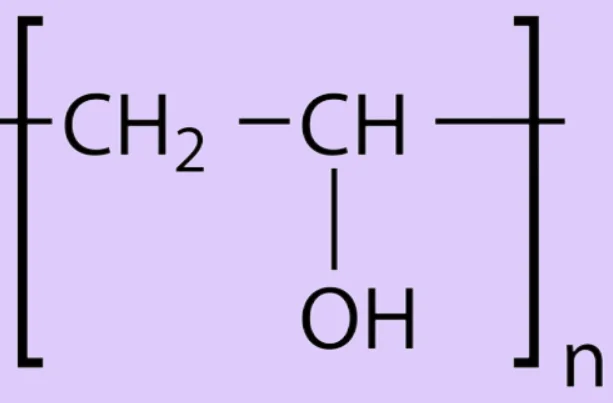
At a molecular level, PVA comprises repeating units of the monomer vinyl alcohol (CH2CHOH). However, because vinyl alcohol is unstable, it cannot be polymerized directly. Instead, PVA is made by polymerizing vinyl acetate (CH2CHOCOCH3) and then converting it to PVA through hydrolysis.
The general formula for PVA can be represented as:
-[CH2-CHOH]-n\text{-[CH2-CHOH]-}_n-[CH2-CHOH]-n
Where N represents the number of repeating units in the polymer chain.
The hydroxyl groups (-OH) attached to the carbon atoms in the polymer backbone are highly polar, significantly influencing PVA’s physical and chemical properties. The degree of hydrolysis, or the percentage of acetate groups replaced by hydroxyl groups, can vary, leading to different grades of PVA with varying solubility, crystallinity, and mechanical properties.
Critical Properties of Polyvinyl Alcohol
The unique structure of PVA gives rise to a range of properties that make it a valuable material in numerous applications. Below are some of the critical properties of PVA:
1. Water Solubility: PVA is known for its excellent water solubility, which is a direct result of its hydroxyl groups. These groups form hydrogen bonds with water molecules, allowing the polymer to dissolve readily in water. The degree of hydrolysis affects the solubility of PVA; fully hydrolyzed PVA is less soluble in cold water but dissolves in hot water, while partially hydrolyzed PVA is soluble in cold and hot water.
2. Film-Forming Ability: One of the most significant properties of PVA is its ability to form strong, flexible films. When dissolved in water and cast into a thin layer, PVA can create a transparent film resistant to oils, greases, and chemicals. This property makes PVA ideal for packaging, coatings, and adhesives.
3. Chemical Resistance: PVA exhibits good resistance to various chemicals, including oils, fats, and organic solvents. This resistance is mainly due to the strong hydrogen bonding within the polymer structure, which provides stability and protection against chemical attack. This makes PVA suitable for use in environments where chemical exposure is a concern.
4. Biodegradability: Another notable property of PVA is its biodegradability. Under the right conditions, microorganisms can break down PVA into environmentally benign byproducts such as water and carbon dioxide. This property is particularly important in applications where environmental impact is a concern, such as packaging materials and single-use products.
5. Mechanical Properties: PVA has excellent tensile strength and flexibility, which can be further enhanced by blending it with other polymers or cross-linking. The polymer’s mechanical properties can be tailored to meet specific application requirements, making it versatile in industries such as textiles, construction, and medical devices.
Applications of Polyvinyl Alcohol
The unique properties of Polyvinyl Alcohol have led to its widespread use in various industries:
- Textiles: PVA is used as a sizing agent in textile production, which helps strengthen and protect yarns during weaving. Its ability to be easily removed by washing makes it ideal for this application.
- Pharmaceuticals: In the pharmaceutical industry, PVA is used in tablet coatings, drug delivery systems, and hydrogels for wound care and tissue engineering.
- Packaging: PVA’s film-forming and water-soluble properties make it an excellent material for creating biodegradable and water-soluble packaging films.
- Construction: PVA is an additive in cement and mortar to improve their flexibility, water resistance, and overall durability.
- Paper and Adhesives: PVA is a binder in paper products and a critical ingredient in adhesives due to its strong bonding capabilities and chemical resistance.
The Future of Polyvinyl Alcohol
As industries continue seeking materials that balance performance and environmental sustainability, PVA is likely to play an increasingly important role. Advances in polymer science are expected to lead to the development of new PVA-based materials with enhanced properties and broader applications. Additionally, the growing emphasis on sustainability and biodegradable materials will likely drive further innovation in using PVA in eco-friendly products.
Conclusion
Polyvinyl Alcohol (PVA) is a unique polymer with a range of properties that make it invaluable in various industries. Its structure, characterized by hydroxyl groups, gives it exceptional water solubility, film-forming ability, and chemical resistance. These properties, combined with its biodegradability and versatility, make PVA a key material in applications ranging from textiles to pharmaceuticals. As research and development continue, the potential for PVA to contribute to innovative and sustainable solutions will only grow, solidifying its place in the future of materials science.

Karen Altizer is a seasoned professional with a wealth of expertise in marketing and communications, adept at crafting compelling narratives and strategic messages tailored to various stakeholders.